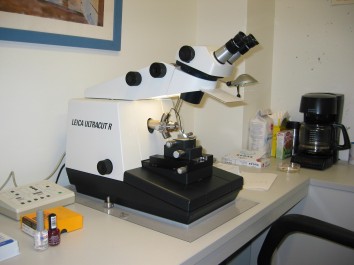
Ultracut Microtome
An ultra-microtome is an essential piece of equipment in any Electron Microscopy (EM) laboratory. Embedded brain tissue is held in the swing arm in the center of the picture above. The tissue is moved forward very slowly and is cut using a glass or diamond knife. The resulting sections of tissue can be as thin as 60 nanometers (nanometers are 1 billion times smaller than a meter). This thickness allows the tissue to be viewed on an EMscope which shoots a beam of electrons through the tissue so that it can be magnified over 40000 times. This level of magnification is necessary for effective analysis of synaptic structure. Training on this piece of equipment is time consuming but mastering this technique is rewarding and valuable on your resume.
–
EM Trimmer
This trimmer allows for very rapid tissue block preparation for the ultra-microtome. Traditionally this trimming work was done by hand using flat razor blades. This took a long time and it was difficult to master the technique. On the trimmer above tissue blocks are held in the arm on the right of the picture and a steel grinding tool is advanced towards the tissue. This grinder spins at very high RPM’s and the resulting waste material is vacuumed away. The resulting trimmer brain tissue blocks are clean and ready for the ultra microtome. Training on this piece of equipment is relatively easy and compliments your efforts on the ultra microtome.
–
Imaging Microscope
This high powered microscope allows for orientation and digitizing of thick sections from the microtome. This microscope is not an EMscope as it uses light instead of electrons. The maximum magnification is only 1000X. A digital camera is mounted to the tube at the top of the microscope and this allows for the capture of magnified images that can be subsequently displayed on the computer that is visible on the right of this picture. The microscope and camera are also relatively easy to master. The imaging software on the other hand takes a lot of time.
–
Cryostat
The cryotome is very similar to the ultracut microtome. It is essentially a microtome within a freezer for cutting thin tissue sections that are frozen. The cryotome sections tissue (usually brain) anywhere from 5 to 100 microns thick so that it can then be prepared for light or confocal microscopy. This is one of the pieces of equipment that students in the Behavioural Neuroscience (PSYC 2605) lab course learn to use.
–
Transmission Electron Microscope
Transmission electron microscopy (TEM) is a microscopy technique whereby a beam of electrons is transmitted through an ultra-thin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto an imaging device, such as afluorescent screen, on a layer of photographic film, or to be detected by a sensor such as a CCD camera.
TEMs are capable of imaging at a significantly higher resolution than light microscopes, owing to the small de Broglie wavelength of electrons. This enables the instrument’s user to examine fine detail—even as small as a single column of atoms, which is thousands of times smaller than the smallest resolvable object in a light microscope. TEM forms a major analysis method in a range of scientific fields, in both physical and biological sciences. TEMs find application in cancer research, virology, materials science as well as pollution,nanotechnology, and semiconductor research.
–
Confocal Microscope
Confocal laser scanning microscopy (CLSM or LSCM) is a technique for obtaining high-resolution optical images with depth selectivity. The key feature of confocal microscopy is its ability to acquire in-focus images from selected depths, a process known as optical sectioning. Images are acquired point-by-point and reconstructed with a computer, allowing three-dimensional reconstructions of topologically complex objects. For opaque specimens, this is useful for surface profiling, while for non-opaque specimens, interior structures can be imaged. For interior imaging, the quality of the image is greatly enhanced over simple microscopy because image information from multiple depths in the specimen is not superimposed. A conventional microscope “sees” as far into the specimen as the light can penetrate, while a confocal microscope only “sees” images one depth level at a time. In effect, the CLSM achieves a controlled and highly limited depth of focus. The confocal microscope at Nipissing University was purchased on a grant by Dr. Ewa Cholewa who graciously allows our lab access.
–
Various Other Microscopes
There are several other light and dissecting microscopes available for use in our facility.
–
Related articles
- Incredible Technology: How to Explore the Microscopic World (livescience.com)
- Asia’s first electron microscope on display (thehindu.com)